Category: Publications
aVaxziPenTM solid dose vaccines are thermostable compared to liquid vaccines
aVaxziPenTM Limited and Sementis Limited have completed a feasibility study formulating vaccinia-based Sementis Copenhagen Vector (SCV) system into aVaxziPen’s solid dose vaccine (SDV), which allows needle-free injection of vaccines using aVaxziPen’s Multi-use pen and disposable cartridge vaccine delivery platform.
The study demonstrated that the SDV formulation retained full vaccine titre for at least 12 months at 4oC and 22oC and higher titre retention at 37oC and 45oC compared to the liquid controls.
Methods
SCV liquid vaccine stock, provided in storage buffer, 10mM Tris, pH 8 was formulated using aVaxziPen’s proprietary formulation containing stabilising and bulking excipients prior to manufacture of solid dose vaccine (SDV). Each SDV containing 1.45×107 plaque forming units (PFU) per dose were packaged in sterile screw cap polypropylene tubes and were stored within secondary packaging (moisture barrier bags) under minimum humidity environmental conditions. Liquid controls were formulated in 10mM Tris pH 8/150mM NaCl targeting the measured titres of the solid doses (1.45×107 PFU/dose). Aliquots were made in sterile O-ring screw cap polypropylene tubes. SDVs and liquid controls were set on stability at different temperatures: 2-8oC, 22oC, 37oC, and 45oC based on ICH Quality Guideline for Stability Testing of New Drug Substances and Products Q1A(R2) with adjustment for available materials, equipment and the pilot nature of the study. Vaccine stability was assessed by measuring vaccine potency by plaque assay. The limit of detection (LoD) was designated as the lowest dilution factor assayed. Where no plaques were detected, a value of 10 was designated for graphical purposes.
Results
Solid dose vaccine formulation of SCV was manufactured using aVaxziPen’s microtableting technology and subjected to stability study for up to 12 months. The thermostability of the viral vaccine in the SDV formulation at the different time points (1, 3, 6, 9, and 12 months) was consistently greater than that of the liquid formulation.
At 2-8oC (Figure 1A), the SDV formulation showed full retention of potency of the viral vector vaccine. The difference in titre between the Day 0 and 12-month samples was minimal (0.06 log10 PFU) for the SDV formulation compared to 0.7 log10 PFU in the liquid control.
At room temperature, 22oC (Figure 1B), the loss of titre in the SDV formulation was less than 0.5 log10 PFU after 12 months compared to more than 3 log10 PFU in the liquid control.
Similarly, at 37oC and 45oC (Figure 1C and 1D), the liquid control lost more than 2 log10 PFU) after 30 days and 7 days of storage, respectively. The vaccine formulated as a SDV had greater retention of titre at these temperatures.
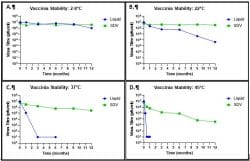
Figure 1: Assessment of retention of infectivity titre of Sementis Copenhagen Virus (SCV) in aVaxziPen’s Solid Dose Vaccine (SDV) stored at, (A) 2-8oC, (B) 22oC, (C) 37oC, and (D) 45oC. Data are shown as PFU/dose where each data point represents the mean ± SEM of doses tested (n=2-3)
Discussion
aVaxziPenTM has produced a solid dose vaccine (SDV) formulation of Sementis’ recombinant vaccinia, SCV, vaccine, which have been shown to be stable at 22℃ for 12 months and have greater stability relative to a standard liquid formulation at 37℃ and 45℃. SCV is a live, attenuated viral vaccine platform that requires infectivity to function as a vaccine. Retention of infectivity is dependent on maintaining the integrity of all the biological components of the virus particle. The stability of vaccines is an important consideration in relation to how it is used and the overall cost-effectiveness of that vaccine. Stability studies are an important aspect of the regulatory assessment. The use of thermostable SDV formulations of such vaccines will facilitate distribution by reducing cold chain requirements, while the additional benefits are provided by the needle-free delivery of the SDV by the reusable, self-actuating aVaxziPenTM device.
Conclusion
aVaxziPen’s solid dose vaccine technology can avoid many of the challenges associated with conventional liquid formulations of vaccines such as restrictive cold-chain requirements and vaccine hesitancy associated with needles. aVaxziPen’s thermo-stable, needle free technology offers the potential to enhance the cost effectiveness of vaccines through reduced resource utilization in their storage, handling and delivery. This study clearly demonstrates that aVaxziPen’s solid dose formulation successfully enhanced thermal stability compared to storage in a liquid formulation.